The goal of regenerative research is to develop the means to restore function in damaged tissues and organs - for example, in accidental injury such as burns, wounds, spinal injury and broken bones, and in disease such as diabetes, Parkinson's disease and other degenerative conditions. This involves the development of natural and synthetic tissues and/ or cells for implantation into the body aimed at replacing, repairing, regenerating, reconstructing or enhancing function.
Regenerative studies may involve the use of cell-derived therapeutics, stem cell therapies, and the use of advanced biomaterials for scaffolds in tissue engineering and implants. Stem cell technologies are a key focus in regenerative studies. By growing stem cells in the laboratory, the hope is to generate tissue-specific cells that can be introduced into the body to help repair organs or to express vital proteins. Any tissue or cell transplant, however, involves a risk of rejection. Rather than taking stem cells from a donor source (adult- or embryonic-derived cell lines) it is possible to carry out somatic cell nuclear transfer, or 'therapeutic cloning' to create stem cells that are genetically matched to the potential recipient.
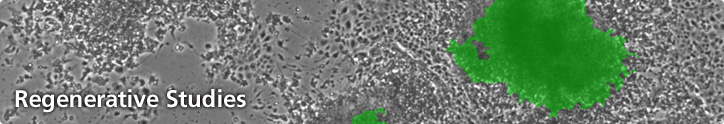
A pluripotent stem cell can remain undifferentiated during sub culture but is capable of differentiating into virtually any type of tissue induced by specific cell culture conditions. Stem cell culture, therefore, requires carefully controlled conditions that promote consistent and healthy cell populations. Microscopy techniques such as brightfield, darkfield, and phase contrast, are routinely used to monitor cell growth in culture. These and additional techniques such as DIC, Hoffman modulation contrast and fluorescence (epifluorescence and confocal) imaging may be used in the identification and characterisation of stem cells. Unique stem cell markers (receptors and other cell surface features), for example, can be visualised using immunofluorescence techniques. Another fluorescence approach involves using stem cells transfected with a GFP reporter which switches-off once cells become differentiated.