External quality assurance (EQA) schemes for medical screening programmes aim to ensure consistently high quality diagnoses. EQA is established in cervical cancer and breast cancer screening and has been implemented in many countries around the world.
Traditionally, EQA involved disseminating sets of test slides to all personnel involved in reading clinical slides (primary screener, checkers and pathologists) the diagnosis of which have been pre-agreed by a panel of experts. Results of these tests are used to assess the performance of both individual screeners and of individual screening centres. While the implementation of such schemes has improved the quality of screening services and focused attention on adequate training and on-going development and assessment of screening capabilities, testing procedures have been the subject of some debate. It is difficult, for example, to compare, the performance of different centres when the slides they read are not identical (tests tend to be carried out on a regional basis). Slides are also frequently broken or lost in transit when being passed from one centre to another.
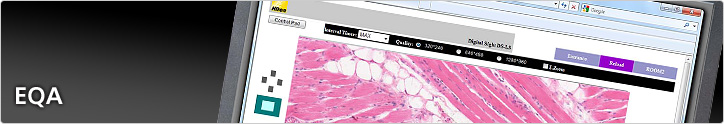
The development of digital imaging, digital slide and telepathology technologies has provided an alternative means of carrying out EQA assessments. Slide digitisation allows slides to be preserved in their entirety and at all magnifications, allowing participants to examine slides on a PC in exactly the same way as they would on a microscope. Image quality is such that all the information available on a 'real slide' is captured in the 'virtual' version. Digital slides remove the need to transport slides around the country and allow participants to take part in EQA tests when it is most convenient to them.