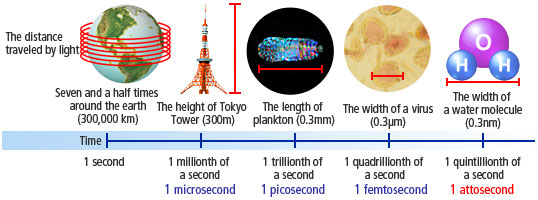
In one second, light travels seven and a half times around the Earth, or 300,000 kilometers. The tiny instant of time that it takes for light to travel a mere 0.3 nanometers*1 is called an attosecond. Creating flashes that only shine for an instant, optical-science research has entered an era in which new types of science that transcend the imagination are possible.
- *1Nanometer: one billionth of a meter
Stanford the industrialist and the photographic sequence

Leland Stanford
©The Granger Collection, New York / PPS
Leland Stanford, the industrialist founder of the Central Pacific Railroad, which was one of the transcontinental railroads linking the east and the west of the United States, lost his only son, who was a student in Europe at the time, to intestinal typhus. Overcome with grief at the loss, he pondered what he might do, and in 1891 he founded Leland Stanford Junior University, making use of land on which he had intended to build a racecourse. Today, Stanford University is renowned as one of the world's foremost universities.
As attested by the fact that he had intended to use his own land to build a racecourse, Stanford, whose legacy includes many magnificent achievements, harbored a great love of horses, and in 1872 he made a wager with a friend on whether all four legs of a horse ever leave the ground at the same time while it is running. Stanford's love of horses was to be the impetus for enormous future progress in science and technology.
Photograph “The Horse in Motion”—the 12-photograph sequence shot by Muybridge. This put an end to the argument by proving that all four legs of a horse leave the ground simultaneously while it is running.
©Akg / PPS
To get to the bottom of this, Stanford commissioned the British photographer Eadweard Muybridge to shoot photographs of a horse running flat out. Employing a technique unknown at the time, Muybridge used 12 cameras to shoot a photographic sequence of a horse as it galloped at full speed. The answer yielded by his work was that all four legs of the horse do leave the ground simultaneously while it is running. Muybridge's photographic sequence was hugely influential on Thomas Edison's invention of the kinetoscope and on the subsequent invention of the cinematic projector, which would become the basis for the development of motion picture film. It is fascinating to see how Stanford's wager spawned a new technology and had an impact that has lasted to this day.
Even today we find a sequence of photographs that capture an instant in time fascinating. A TV picture consists of a stream of still images, about 0.03 seconds apart. How short an instant are we able to capture, and what will we see when we capture a single instant in the realm of the microscopic?
During the twentieth century, the quest to “see the invisible” was pursued, and in the realm of the microscopic, the development of the microscope allowed viewing at the level of the atom. Using techniques akin to a photographic sequence, attempts are now being made to understand motion in a single instant at the atomic and electron level.
Artificial light—the laser

Figure. The difference between natural light and laser light. In a laser, the peaks and troughs of the waves are uniform, as is the direction of the light.
The motion of an atom or an electron is captured and viewed as a photographic sequence. Since these particles move at virtually the speed of light, viewing them requires a flash that is shorter in duration than has ever been known. A flash that shines for the shortest time interval possible can be generated using a laser.
The laser is an artificially generated light that does not occur in the natural world. The characteristics of a laser are that its wavelengths (the length of the light waves) and its phase (its peaks and troughs) are all in unison and that the light all points in the same direction. Naturally occurring light does not exhibit these properties, as it combines light of differing wavelengths and phases. This twentieth century invention changed the world, and applications of the laser now include the laser printer, the read heads in CD and DVD devices, and of course the laser scalpel used in medicine.
It was the physicist Albert Einstein, famed for his theory of relativity, who first showed how light with these characteristics could be artificially generated. In 1917 he proposed the concept of “stimulated emission” as follows: light striking matter causes some electrons to briefly gain energy, and when these electrons return to their previous energy levels, light in phase is generated. The term “laser” is an acronym for “light amplification by stimulated emission of radiation.”
Even after this theory had been postulated, it was some time before the laser was put into in practice. In 1954, the American physicist Charles Townes succeeded in implementing the “maser” —which stands for “microwave amplification by stimulated emission of radiation,”— in which stimulated emission is used to generate microwaves. Although the laser and the maser are identical in terms of their light characteristics, their respective wavelengths differ. The very same year, University of Tokyo physicist Koichi Shimoda established his theory on the amplification of light of uniform wavelength, on which the laser would be based, and this theory represented the foundation for the implementation of the laser in 1960.
Nowadays it is possible to generate lasers of any wavelength. More and more applications are being enabled by laser oscillation in the ultra-violet and X-ray spectra, as well as in the far-infrared, near-infrared, and optical spectra. Once laser oscillation became possible, a whole raft of technologies flowered, and the significance of the laser is that it is the key underlying technology for all of them.
According to laser specialist Katsumi Midorikawa, a senior research fellow at RIKEN (The Institute of Physical and Chemical Research), the next objective is maximum-speed stroboscopic photography using lasers. How well can we capture and view the motion of atoms and electrons using the fastest stroboscopic photography available today?
Viewing the motion of an electron
Observing an object as it changes moment by moment and verifying the reality of what is happening in the process-as Muybridge did when he shot his photographic sequence of the horse-is the very basis of science. It was in the 1990s that man was first able to generate flashes lasting a picosecond (one trillionth of a second). In the 2000s this has been reduced by a factor of 1000, and the use is now possible of optical light flashes that last a femtosecond (one quadrillionth of a second). The use of femtosecond flashes should enable viewing of chemical reactions in matter frame-by-frame-like the photographs of a milk crown. For Dr. Midorikawa and his team, the challenge continued as they sought to develop the attosecond pulse (a pulse lasting one quintillionth of a second).
“It is the attosecond pulse that will enable us to see the motion of an electron.”
Dr. Midorikawa's team is skilled at generating high-intensity 1-attosecond pulses that are only possible with lasers. If the intensity of the pulses is high, the electron absorbs lots of photons, enabling their motion to be seen more directly. It is no exaggeration to say that it is the motion of electrons that determines the properties of matter. If the motion of electrons were visible, this would doubtless lead to revolutionary developments in technologies at the very foundations of industry, such as semiconductors, super-conductivity and ferro-magnetism.
However, electrons move at high speed-almost at the same speed as light. Photographing them would require an attosecond pulse rather than a femtosecond pulse. To generate a pulse of extremely short duration, such as one attosecond, the wavelength of the original light used to generate the laser must also be short. Using optical light, pulses have only ever been generated in the femtosecond region. Generating an attosecond pulse requires the superimposition of light from what is called the “soft X-ray” spectrum, which is shorter in wavelength.
Dr. Midorikawa's group directed an extremely strong optical laser at matter, and from this, light of various wavelengths in the soft X-ray spectrum (high-order harmonics) was generated. An accumulation of these short wavelengths of light produces an attosecond pulse.
The Midorikawa group is proud of the fact that, while many groups are studying attosecond pulse, its attosecond pulse has the highest intensity.
Intensities some 1 billion times higher than large-scale synchrotron radiation can only be achieved by laser. If the motion of an electron is to be viewed, the electron must gain a sufficient level of energy, and this requires a high-intensity pulse. In 2006, using the highest intensity attosecond pulses in the world, Dr. Midorikawa successfully observed the scattering of electrons that had gained sufficient energy (the so-called nonlinear optical phenomenon). This was the result of a 15-year effort. “This phenomenon can be confirmed only by light that has a uniform direction, phase and wavelength. I felt that soft X-ray laser had at last come of age,” Dr. Midorikawa said.
Once the attosecond pulse had been generated, the next step-viewing the motion of an electron-required even further ingenuity. It is difficult to view the motion of an electron in situ using a microscope. Accordingly, the Midorikawa team directed a stream of light flashing at attosecond intervals at a mixture of the gases, neon and helium, and used a spectroscope to observe how electrons separated from atoms of the two gases for extremely small intervals of time, before returning to their original positions again.
These electrons, which flew out from two different atoms at virtually the same time and in virtually the same place, emitted light when they returned to their atoms, and there was a slight time lag between these light emissions. It was this time lag that created an interference fringe as light with differing wavelengths-generated when the electrons returned to their respective neon and helium atoms-overlapped and enabled the motion of the electrons to be observed. This time interval was approximately 690 attoseconds in duration. This was achieved in 2007 and represented the first successful attempt to capture the motion of electrons using attosecond pulses.
What will attosecond pulses reveal in the future?
Although the attosecond pulse is now a reality, it is not yet clearly understood what will be possible in practice, as there is vast potential.
One area that is currently attracting attention is technology for observing living cells moment-by-moment. The soft-X-ray wavelength region is known as the “window on water,” as it allows good observation of substances that contain water. Ordinary soft X-rays are absorbed by such substances, but soft X-rays with special wavelength penetrate them and high resolution can be achieved. This technology would resolve both the problems that have been encountered so far-namely, that cells die when X-rays or ultra-violet rays, which biologists have thought necessary for attaining high resolution, are used, and that atomic-level resolution cannot be achieved if visible light, which is weaker than X-rays and ultra-violet light, is used to observe still-living cells.
For example, in the past, the only way to investigate the way in which cells infected with malaria undergo change was to dehydrate them, that is, the cells would be dead. Since the cells had been frozen, this was not really a proper investigation of change over time, as it was impossible to track how the frozen cell changed over time. Using a laser with attosecond pulses, however, would enable detailed moment-by-moment observation of living cells.
Innovative optical-science research is currently progressing in the terahertz region*2 as well as in attoscience. Dr. Midorikawa is attempting to combine these two areas and to pursue development in virgin territory in the field of light under the banner of the Extreme Photonics project.
- *2Terahertz region: long-wave light with wavelengths of the order of 100 micrometers. Used in astronomy and advanced imaging technology.
“There are no limits in optical science-just the horizon,” Dr. G. Mourou said in 2008.
Dr. Midorikawa was moved by these words. Although electron motion can now be measured, very little is known about atomic orbitals and electron clouds. “Next time,” says Dr. Midorikawa, “I want to capture the movement of electrons like a movie.” Optical science will likely continue to confound conventional wisdom and to show us the world in a new light.
Collaboration on materials / Date of article posted
Dr. Katsumi Midorikawa Senior research fellow at RIKEN (the Institute of Physical and Chemical Research) / September 2009

