Researcher TestimonialsIkuo Wada, Ph.D.
Department of Cell Science, Institute of Biomedical Science, Fukushima Medical University

Dr. Wada (right)
1. What kind of research are you engaged in?
I have long been interested in the mechanism by which secreted proteins are efficiently produced. To study this, I have conducted a series of studies into the dynamics of inner membrane proteins and the dynamics of cargo protein molecules themselves. After my biochemistry research, I started fluorescence imaging, and then went on to use single molecule techniques, such as FCS (fluorescence correlation spectroscopy) and single molecule tracking. Because I want to understand what makes "living" cells, I have a boundless interest in changes in cell structure and in the dynamic behavior of multiple molecules.
2. Why were you interested in the N-SIM super resolution microscope?
Initially, I anticipated that I would be able to observe the microstructures of endomembranes that cannot be recorded with a confocal microscope. When I used it in practice, I discovered that this was not the only thing I could now do—and when I used it in combination with an A1Rsi confocal microscope system, it felt as if a whole new world had been opened up.
3. How did you find the N-SIM to use in practice?
It was extremely user-friendly and easier to use than I had imagined. It is simple to modify the analysis parameters, and the fact that it allows an intuitive understanding of the correlation between these parameters and the results of data acquisition makes it easy to use. Just modifying the parameters a little could eliminate the known artifacts of SIM. As all the original acquisition data are preserved, it is so easy to go back to the original and confirm that the reconstructed images are correct.
I was also particularly struck by the superb resolution in the Z-axis direction. Also, I was surprised to find that scanning with the N-SIM unit produces much less photobleaching than with a confocal LSM unit at the same magnification. The N-SIM unit is now our first choice.
4. How have you been using the A1Rsi and the N-SIM in combination with one another?
I mainly use the N-SIM for observing cell structures as still images. On the other hand, the A1Rsi can perform ultra-high-speed image acquisition and allows FRAP and photo stimulation. Unless both devices are used, the information we can obtain is limited. The combination of N-SIM and A1Rsi allows us to acquire high-resolution images of specific structures. This is because A1Rsi highlights and activates only regions labeled with photoactivatable proteins such as PA-GFP. The ability to highlight only specific structures represents an extremely powerful capability.
5. What are the advantages of connecting analysis units such as the TCSPC unit to the A1Rsi confocal microscope system?
First, the A1Rsi is highly extensible and operations, including external inputs, are extremely simple. This makes it easy to use—even for an amateur of optics like me. We use FLIM information obtained with the TCSPC unit for analyzing the FRET efficiency. Although there are sometimes difficulties with the acceptor-bleaching method, with FLIM, results are obtained quickly with few artifacts appearing, making it useful for FRET measurements when, for example, studying structural changes in molecules. Photon static information, including FLIM and FCS/PCH information, from living cells obtained with the TCSPC unit supplements detailed structural information obtained with the N-SIM. Nikon’s 60x water-immersion objective lens is the default lens for the TCSPC unit, while 40x is the default objective for other manufacturers. Nikon optical systems are surprisingly high-performance and give much higher photon counts per molecule than those acquired with other manufacturers’ optics. This is probably due to the extremely high brightness of Nikon objective lenses.
6. What developments would you like to see for N-SIM in the future?
Firstly, faster image acquisition and simultaneous acquisition of two colors. Even without two cameras, if it could offer dual-view display with just a single camera, this would be quite effective—even with only 256 pixels. Users can probably be divided into those who want to view a larger area, and those who want to see faster change.
Secondly, improvements in illumination pattern contrast and a better CCD camera with a lower dark current may help improve reconstruction of dark (noisy) samples and should lead to wider use of the N-SIM in cell biology.
In addition, I feel that successful application of the N-SIM depends on proper imaging techniques and knowledge. I hope that Nikon is involved in promoting optical imaging education.
7. Please describe your impressions of Nikon.
Nikon’s engineers have been giving thorough consideration to my requests. Instead of responding to my wishes by saying “it can’t be done,” they are properly considering how they can bring them to fruition. Nikon products are also outstanding in terms of system extensibility. It seems to be difficult to attach third-party peripheral devices to other manufacturer’s products, particularly in Japan.
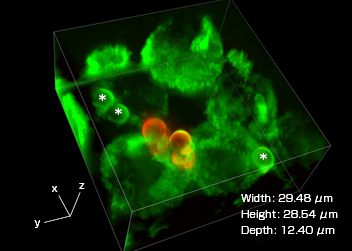
Three-dimensional image of phagosomes taken with a super resolution microscope
J774/mVenus-SNAP-23 cells observed using a super resolution microscope (Nikon’s N-Structured Illumination Microscopy System). Phagosomes (highlighted with green fluorescence), which incorporate beads wrapped in mVenus-SNAP-23 (which are marked with a *), are visible in the cells. After Phagocytosis, the addition of an Alxa594-labeled antibody that recognizes the IgG used in opsonization allows confirmation that there are beads outside the cells (highlighted with red fluorescence).

System:
N-SIM + A1Rsi + TCSPC (time-correlated single photon counting) unit (third-party peripheral device attached to the A1)
