
Chances are you learned about photosynthesis in high school. Structures in a plant’s leaves convert the energy of light from the sun into chemical energy and store it as carbohydrates. Plants need only solar light energy, water (H2O) and carbon dioxide (CO2) to produce carbohydrates. This process occurs in chloroplasts, which utilize a green pigment called chlorophyll. If we could learn to replicate this process, we might be able to harness sunlight — the ultimate unlimited energy — as an energy source.
Unraveling the mysteries of photosynthesis
“Photosynthesis is the most important infrastructure for human activities,” says Professor Nobuo Kamiya, a researcher of biological structural chemistry at the Osaka City University Advanced Research Institute for Natural Science and Technology (OCARINA). “It supports the life of organisms that breathe oxygen, and enables production of foodstuffs, clothing, houses, cars, other industrial products and many other chemical compounds we use in our everyday lives.”
Your high school science teacher probably gave you only a very rough idea of how photosynthesis works. First, there are light reactions, in which the plant uses the captured solar energy to split water and generate biological substances, while releasing oxygen molecules (O2). Next, in dark reactions, which do not require light, the plant absorbs carbon dioxide from the air and converts it to carbohydrates, using the chemical energy stored in the biological substances from the previous phase. Complicated processes, these two stages of photosynthesis involve more than 100 enzymes working together as catalysts.
Scientists have deciphered most of the mechanics of photosynthesis over the last 200 years, but there remained one mystery: the structure and reaction mechanisms of a large membrane protein called Photosystem II (PSII) that uses solar energy to split water into oxygen molecules, hydrogen ions (H+) and electrons (e-). Dr. Kamiya and his team discovered that a cluster which plays the crucial role in this reaction — the catalytic center — comprises four manganese (Mn) atoms and one calcium (Ca) atom linked by five oxygen (O) atoms. They designated it the Mn4CaO5 cluster.
“Having discovered the structure and composition of this,” Dr. Kamiya explains, “we have now amassed sufficient knowledge to realize artificial photosynthesis. If we can create a catalyst that generates oxygen by mimicking this structure, it would be a huge step toward practical application of artificial photosynthesis.”
Artificial photosynthesis: a solution to the energy crisis?
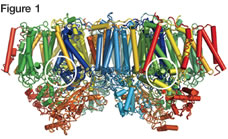
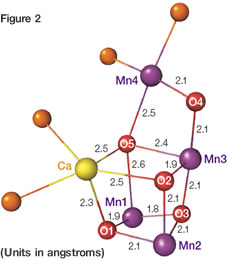
Figure 1 is the schematic diagram of the molecular structure of Photosystem II (PSII), which catalyzes the reaction: 2H2O→O2+4H++4e-. The white circles indicate the locations of the catalytic center, the Mn4CaO5 cluster (Figure 2). The cluster is unique because the oxygen atom No. 5 (O5) is connected with three Mn atoms and one Ca atom. As a result, the connective distances to O5 are longer than the others, creating a structure that resembles a distorted chair.
(Illustrations are courtesy of Dr. Nobuo Kamiya)
Some researchers say that, ideally, if we could harness the power of just one hour of sunlight that reaches Earth’s surface, we could meet current global energy needs for one year. Solar panels are at work today, but it is nature’s process — photosynthesis — that holds staggering promise.
Humans rely almost exclusively on finite sources of fossil fuels for energy, and processing this releases carbon dioxide, a gas that contributes to the greenhouse effect. Artificial photosynthesis could break our dependence on fossil fuels, Dr. Kamiya says.
Revealing the whole process of photosynthesis, including the discovery of the Mn4CaO5 cluster, therefore, was crucially important, because artificial photosynthesis can harness the unlimited energy of sunlight, along with carbon dioxide, to produce chemical compounds, such as methanol, formic acid and hydrogen (H2), that can be used as energy sources and industrial materials. “This will lead to the solution of some of Earth’s environmental problems. We must proceed, believing that we can develop this for practical use someday,” Dr. Kamiya says.
Dr. Kamiya and many other researchers continue to search for solutions. Osaka City University is building the Artificial Photosynthesis Center, an institution for academia and industry to collaborate in research with the goal of starting methanol production through artificial photosynthesis in 2030.
“We hope to replace fossil fuels with chemicals, such as methanol, generated using a combination of solar power, water and carbon dioxide by artificial photosynthesis,” Dr. Kamiya says. “With this technology, we could utilize twice as much carbon dioxide as plants do for photosynthesis for a variety of applications, and produce energy equivalent to an amount 10 billion people could use for 1,000 years. The energy crisis would no longer be a problem.”
- 1. Plant power: tapping into truly green energy
- 2. Biomimetics: recreating structures found in nature
- 3. Hibernation: the possibility of a longer lifespan

